Crystal Growth Mystery of Diamond
The process of crystal formation is the process
Subcategory: Company Information
Subcategory: Industry Information
2022-01-10
The process of crystal formation is the process by which a substance changes from another phase to a crystalline phase. That is, the original amorphous material under certain physical and chemical conditions (temperature, pressure and component concentration, etc.) into a crystalline material process. The main ways of crystal formation are:
A gas at its supersaturated vapor pressure or supercooled temperature is directly converted from the gas phase to crystals. The most typical example of life is ice flowers. The ice flowers on the glass windows in winter are the result of direct crystallization of water vapor in the air.
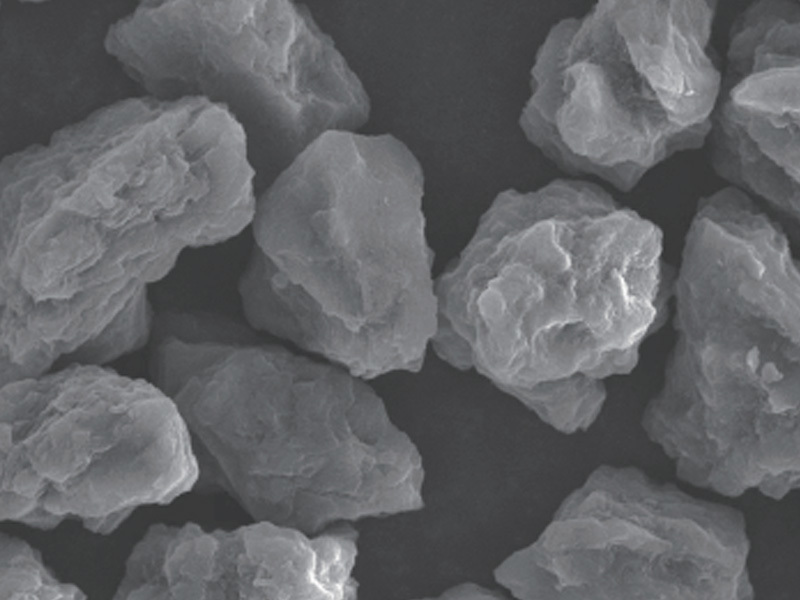
diamond single crystal, fine grain diamond, synthetic diamond, diamond
Crystallization from a liquid (solution or melt)
from solid phase to crystal
This phase change can also be in two ways:
Because the amorphous body has a larger free energy than the crystalline body, it can spontaneously transform to a crystalline body with a smaller free energy. Such as graphite products have amorphous coke, asphalt, etc. after high temperature action to form graphite crystals.
This phase transition is commonly referred to as the homogeneous multimodal transition. Synthetic diamond, for example, is usually made by the transformation of graphite at high temperatures and extreme pressure.
diamond single crystal, fine grain diamond, synthetic diamond, diamond
But in the actual growth process, due to the growth of the external environment is always more or less deviated from the ideal conditions, the surface network in the growth can not strictly layer by layer parallel to the outward, but often according to the lattice structure of the polymerization of the particle group into a group of adhesion to the crystal nucleus (bud), it is worth noting that between these particle groups and the crystal nucleus (bud), between the particle group and the particle group, not strictly parallel to each other in accordance with the law of lattice structure, there is often a very small angle deviation, and sometimes even cause some defects in the crystal structure. In addition, as the crystal grows, the unevenness of solute supply will gradually become obvious. Relatively speaking, the corner top has the most chance to receive solute, followed by the crystal edge, and the center of the crystal plane is the smallest. Therefore, the mass points will preferentially accumulate near the corner top and the crystal edge.
As for the mechanism of transforming one crystal into another crystal, we take graphite into diamond as an example. Due to the limitation of difficult detection under high temperature and high pressure, we cannot intuitively understand the change process, so its mechanism is inferred by researchers according to the general law of crystal, and there are many kinds of statements, among which the most important theoretical models include two types: one is not (no diffusion, direct transformation point of view), including solid phase transformation theory and structural transformation theory; The other is (dissolution and diffusion point of view), including solvent theory, catalyst theory, solvent-catalyst theory, etc.
It is believed that the chemical bonds of graphite are first broken and then reconstructed into diamond bonds, which have undergone a process from the opening of carbon atomic bonds to the recombination of new bonds. This is called a reconstructive transition in crystallization chemistry. Achieving this transformation often requires the provision of more energy. In this process, several steps including the dissolution, diffusion and recrystallization of carbon atoms can generally be considered as a solid-liquid-solid transition, following the general law of crystallization from the liquid phase mentioned above. For example, crystals grow up with time, and the surface has growth steps and growth spirals.
Now on the current more popular large particle diamond synthesis mechanism from the solvent to explain the mechanism:
The driving force of diamond crystallization is the supersaturation of diamond in solution. The crystal morphology and the formation and growth of crystal are directly related to the supersaturation. The degree of supersaturation depends on the difference between the solubility of graphite and diamond under specific P and T conditions. The reason for supersaturation is the thermodynamic potential difference between graphite and diamond-the difference between chemical positions. In a certain temperature range, diamond is more stable than graphite, so graphite is easier to dissolve, that is, graphite solubility is larger than diamond. Strong evidence for this theory is the morphology and defects on the surface of synthetic diamonds, such as growth steps and spirals found on the (111) surface of the diamond.
Related Information

Warm winter, warm companion - elements, love and responsibility go hand in hand
At the end of the year, the Spring Festival is approaching, Zhang Yan, general manager of Element, led the volunteers of Element to purchase rice, flour, grain and oil and rushed to nursing homes, orphanages, and poor families to bring a touch of warmth to this cold winter.
Learn More2024/06/27